Zinc Ash
Description of Zinc Ash
Zinc Ash / Zinc dust: 45% 60% 70% 65% 75% 80% 85%
Chemical Properties
Zinc powder is insoluble in water but soluble in acid, alkali and ammonia water.
Has strong performance of reducing action and it can releases hydrogen in the reaction with acid or alkali.
Is easy to form lumps due to dampness. It is easy to produce white basic carbonate on its surface in the wet air, but stable in the dry air.

Application of Zinc Ash
1. Zinc powder is the main raw materials of paint.
2. Coating and chemical products such as sodium zincate, zinc oxide as well as the reducing agent in organic synthesis.
3. Besides, zinc powder is widely used in metallurgical industry (electrolytic zinc), battery industry as well as pesticide, feed, dyestuff and manufacturing industry.
Storage
Stored in dry, airy warehouse without acid, alkali and inflammables. Be cautious of moisture, water and fire during storing and transportation.
Packaging & Shipping
Package
50kg barrel drum or plastic woven bags lined with polythene bags, inside with plastic coating bags or 1000kg big bags as customer required.

Sodium Hydroxide
Sodium Hydroxide Definition

Sodium hydroxide, also known as lye and Caustic Soda is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates NaOH·nH2O. The monohydrate NaOH·H2O crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available “sodium hydroxide” is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, it is frequently utilized alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.
NaOH or Sodium Hydroxide or lye are thin white sheets that are moisture absorbent and soluble in water. It is derived from the evaporation of liquid Sodium Hydroxide, which is itself produced through membrane technology.
Sodium hydroxide or Lye with NaOH chemical formula, a solid white solid with a melting temperature of 2°C and a density of 0.5 Perk profits can easily absorb moisture from the air and, therefore, have to be protected during transportation.
Chemical formula: NaOH
Analysis: 98±1 %
Scientific name: Sodium hydroxide
Industrial Name: caustic soda, lye
Sodium Hydroxide Application
1. Used in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents.
2. Used in as a drain cleaner.
3. Used in the paper industry.
4. Used in the manufacture of sodium salts and detergents.
5. Used for pH regulation.
6. Used in the Bayer process of Aluminum production.
7. Used in many scenarios where it is desirable to increase the alkalinity of a mixture, or to neutralize acids.
8. Used as an additive in drilling mud to increase alkalinity in bentonite mud systems.
9. Poor quality crude oil can be treated with Lye to remove sulfurous impurities in a process known as caustic washing.
10. Widely used in pulping of wood for making paper or regenerated fibers.
11. Used to digest tissues, as in a process that was used with farm animals at one time.
12. Acts as an agent to make the solution alkaline, which aluminum can dissolve in.
13. Traditionally used in soap making.
14. Used as a catalyst for the transesterification of methanol and triglycerides.
15. washing or chemical peeling of fruits and vegetables, chocolate and cocoa processing, caramel coloring production, poultry scalding, soft drink processing, and thickening ice cream.
Sodium Hydroxide Characteristics
1. It is a white solid which has a melting point of 591K
2. It is a stable compound.
3. NaOH is bitter in taste and has a soapy feel to it.
4. It is highly soluble in water and moderately soluble in alcohol.
5. Sodium hydroxide is strongly alkaline in nature.
6. Boiling Point : 1388°C.
7. Melting Point : 318°C.
8. Easily soluble in cold water, hot water.
9. Density : 2.1 g/cm³ @ 25°C.
10. Vapor Pressure : 0 mmHg (approx)
Specification
| Product name | Caustic Soda Flakes 98% | |
| CAS | 1310-73-2 | |
| Standard | Result | |
| NaOH | 98% min | 98.15% |
| Na2CO3 | 0.90% max | 0.60% |
| NaCl | 0.15% max | 0.06% |
| Fe2O3 | 0.007% max | 0.006% |
Packing of Sodium Hydroxide
| Packing: | 25Kg Bags, 24MT Per 20′ FCL |
| Payment: | Negotiable |
| Minimum Order: | 1 x 20′ FCL |
Chemical Information
| Chemical Formula | NaOH |
| CAS number | 1310-73-2 |
| EC number | 215-185-5 |
| UN number | 1823 |
| Molar Mass | 40.0 g/mol |
| Color | White |
| Appearance | White Flakes |
| Melting point | 323 °C |
| Boiling point | 1390 °C |
| Solubility in Water | 111 g /100 ml at 20 °C |
| Synonyms: Caustic Soda, lye |
Caustic Soda Liquid
Caustic Soda Liquid Description

Caustic Soda Liquid, also known as lye and Sodium hydroxide, is an inorganic compound with formula NaOH. Caustic soda uses electrolysis method which result in pure liquid caustic-soda and free of metal impurities, and are marketed in two types: 30% -33% and 48% -50%; which are according to ISIRI 364 Standard and conventional caustic-Soda of industrial consumer units. It is a white ionic compound consisting of sodium cations Na+ and hydroxide anions OH–.
Sodium hydroxide is a highly caustic base and alkali, that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates NaOH·nH2O. The monohydrate sodium hydroxide (NaOH·H2O) crystallizes from water solutions between 12.3 and 61.8 °C.
The commercially available “sodium hydroxide” is often this monohydrate, and published data may refer to it instead of the anhydrous compound.
Sodium hydroxide is used in many industries in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents and as a drain cleaner.
Sodium hydroxide can form several hydrates(NaOH·nH2O), which result in a complex solubility diagram that was described in detail by S. U. Pickering in 1893. The known hydrates and the approximate ranges of temperature and concentration (mass percent of NaOH) of their saturated water solutions are:
Hepta hydrate, NaOH·7H2O: from -28 °C (18.8%) to -24 °C (22.2%).
Penta hydrate, NaOH·5H2O: from -24 °C (22.2%) to -17.7 (24.8%).
Tetra hydrate, NaOH·4H2O, α form: from ?17.7 (24.8%) to +5.4 °C (32.5%).
Tetra hydrate, NaOH·4H2O, ß form: metastable.
NaOH·3.5H2O: from +5.4 °C (32.5%) to +15.38 °C (38.8%) and then to +5.0 °C (45.7%).
Tri hydrate, NaOH·3H2O: metastable
Di hydrate, NaOH·2H2O: from +5.0 °C (45.7%) to +12.3 °C (51%).
Monohydrate, NaOH·H2O: from +12.3 °C (51%) to 65.10 °C (69%) then to 62.63 °C (73.1%).
Early reports refer to hydrates with n = 0.5 or n = 2/3, but later careful investigations failed to confirm their existence.
The only hydrates with stable melting points are NaOH·H2O (65.10 °C) and NaOH·3.5H2O (15.38 °C). The other hydrates, except the metastable ones NaOH·3H2O and NaOH·4H2O (ß) can be crystallized from solutions of the proper composition, as listed above. However, solutions of NaOH can be easily super cooled by many degrees, which allows the formation of hydrates (including the metastable ones) from solutions with different concentrations.
For example, when a solution of NaOH and water with 1:2 mole ratios (52.6% NaOH by mass) is cooled, the monohydrate normally starts to crystallize (at about 22 °C) before the dihydrate. However, the solution can easily be supercooled down to -15 °C, at which point it may quickly crystallize as the dihydrate. When heated, the solid dihydrate might melt directly into a solution at 13.35 °C; however, once the temperature exceeds 12.58 °C, it often decomposes into solid monohydrate and a liquid solution. Even the n = 3.5 hydrate is difficult to crystallize because the solution super cools so much that other hydrates become more stable.
A hot water solution containing 73.1% (mass) of NaOH is an eutectic that solidifies at about 62.63 °C as an intimate mix of anhydrous and monohydrate crystals.
A second stable eutectic composition is 45.4% (mass) of NaOH, that solidifies at about 4.9 °C into a mixture of crystals of the dihydrate and of the 3.5-hydrate.
The third stable eutectic has 18.4% (mass) of NaOH. It solidifies at about -28.7 °C as a mixture of water ice and the heptahydrate NaOH·7H2O.
When solutions with less than 18.4% NaOH are cooled, water ice crystallizes first, leaving the NaOH in solution.
The α form of the tetrahydrate has density 1.33 g/cm3. It melts congruously at 7.55 °C into a liquid with 35.7% NaOH and density 1.392 g/cm3, and therefore floats on it like ice on water. However, at about 4.9 °C it may instead melt incongruously into a mixture of solid NaOH·3.5H2O and a liquid solution.
The ß form of the tetrahydrate is metastable, and often transforms spontaneously to the ? form when cooled below -20 °C. Once initiated, the exothermic transformation is complete in a few minutes, with a 6.5% increase in volume of the solid. The ß form can be crystallized from supercooled solutions at -26 °C, and melts partially at -1.83 °C.
The “sodium hydroxide” of commerce is often the monohydrate (density 1.829 g/cm3). Physical data in technical literature may refer to this form, rather than the anhydrous compound.
The monohydrate crystallizes in the space group Pbca, with cell dimensions a = 1.1825, b = 0.6213, c = 0.6069 nm. The atoms are arranged in a hydrargillite-like layer structure /O Na O O Na O/… Each sodium atom is surrounded by six oxygen atoms, three each from hydroxyl anions HO– and three from water molecules. The hydrogen atoms of the hydroxyls form strong bonds with oxygen atoms within each O layer. Adjacent O layers are held together by hydrogen bonds between water molecules.
Lye Liquid Uses
Caustic-soda, or sodium hydroxide, mostly used by industry and chemical manufacturing companies. Some fields caustic-soda use for:
Refineries Alumina
plastic wrap
Soaps and cleaners
Detergent
Textile processing
Oil refining
Water treatment
Metal processing
Caustic-Soda Liquid Formula
Caustic-soda / Sodium hydroxide ( NaOH), purity / mass: Min. 50.50
Chlorides / NaCl, purity / mass: Max. 0.10
Caustic-soda / Sodium carbonate / Na2CO3, purity / mass, Max. 0.40
Caustic Soda Liquid Specifications
| LYE ANALYSIS | Value |
|---|---|
| NaOH | 98% Min |
| NaCO2 | 1% Max |
| NaCl | 600 ppm Max |
| Ni | 3 ppm Max |
| Fe | 5 ppm Max |
| SiO2 | 100ppm Max |
| Na2SO4 | 0.01% Max |
| Hg | Not Detected |
| PHYSICAL SPECIFICATION | Value |
|---|---|
| Visual Shape | White Flakes |
| Boiling Temperature | 1390° C |
| Freezing Point | 318.4° C |
| Specific Gravity | 2.13 at 25°C |
| Solubility in water | 1g / 0.9 ml water |
Lye Liquid Packing
Bulk, IBC Tank, Drum.
Caustic Soda Flake
Caustic Soda Flake Description

Caustic Soda Flake (sodium hydroxide flake) are prepared by liquid caustic soda which is produced by membrane cell technology. Therefore, this product has the best quality and is without heavy metal impurities. Caustic soda flake are highly hygroscopic and soluble in water and are used in a lot of different industries .The purity of this product is min %99.
Sodium hydroxide (NaOH), also known as lye and caustic soda, is an inorganic compound. It is a white solid and highly caustic metallic base and alkali salt which is available in pellets, flakes, granules, and as prepared solutions at a number of different concentrations. Sodium hydroxide forms an approximately 50% (by weight) saturated solution with water. Sodium hydroxide is soluble in water, ethanol and methanol. This alkali is deliquescent and readily absorbs moisture and carbon dioxide in air.
Caustic soda flake is produced from soda lye in from liquid form (membrane electrolysis) at the flaking plant built on the license of the Swiss company Bertram’s. Caustic soda is an inorganic chemical compound belonging to the group of the strongest alkalis; Caustic soda is highly hygroscopic and soluble in water and alcohols. Caustic soda flake is one of the main raw materials in the chemical industry. Sodium hydroxide is used in most of industries, for example: cosmetics, petrochemical, metallurgical, textile, food or in water treatment. High quality of our product as well as efficient and flexible logistic service allows us to become a preferred supplier throughout Europe and also at overseas markets such as Asia, Africa and South America.
Lye flake (technical sodium hydroxide flakes) – flake mass of white color, very hygroscopic, very soluble in water and alcohol. The formula is NaOH. Technical sodium hydroxide flakes are obtained by evaporating of liquid caustic soda А grade.
Caustic Soda Flake Applications
Caustic Soda Flake is used in organic synthesis processes, petroleum products refining, textile industry in production of viscose silk and in bleaching fabrics, in paper and aniline industry, soap making, production of aluminum and sodium metal, soluble glass, alkaline accumulators, Trilon B.
Caustic soda flakes are transported by railway and motor transport. 25 kg, 50 kg polypropylene bags, special soft containers with a maximum mass of 1,000 kg, 60 kg polyethylene barrels (without the insert) are used for packaging the product. Transportation in 20 and 40-feet sea vans is possible for countries outside the CIS.
Caustic Soda, Lye is one of the most widely used chemicals in the industry. Caustic soda is a commitment, we guarantee a high service level towards our customers.
Worldwide, the major users of caustic soda are the aluminum industry, pulp & paper and the chemical industry. The main applications are water treatment and water purification, as cleaning agent, or a wide range of uses in chemical industry like starch production or for the desulphurization in the petrochemical industry.
solution of Sodium hydroxide (NaOH) in water. Caustic soda is a strong base with a wide range of applications in different industries. We produce caustic soda together with chlorine and hydrogen from the electrolysis of salt brine. With our production in five plants in Europe and our quality
LYE Flake Packing
We have two kind of packing for caustic soda flake, First Lye flake packing is 25kg PP/PE bags in Jumbo Bag (per jumbo bag is weight is .250MT). The next packing form is: 25kg PP/PE shrinking on pallet.
LYE Uses
Caustic soda flake , or sodium hydroxide, mostly used by industry and chemical manufacturing companies. Some

fields caustic soda use for :
– Refineries Alumina
– plastic wrap
– Soaps and cleaners
– Detergent
– Textile processing
– Oil refining
– Water treatment
– Metal processing
we are exporting flake Lye to many destinations all over the world, such as Middle east, African and Europe countries, by the best quality and the most competitive price.
Caustic Soda Flake Formula
Flake Lye is knowing by some name and formula according to application in different industries, such as:
Sodium hydroxide ( NaOH), purity / mass: Min. 99.50
Chlorides / NaCl, purity / mass: Max. 0.10
Sodium carbonate / Na2CO3, purity / mass, Max. 0.40

Sulphur
Sulphur Description

Sulphur (Sulfur) is one of the most important agricultural and industrial raw materials and is considered a strategic product sulfur is an odourless, tasteless and polyvalent nonmetal which is mostly in the form of yellow crystals and is obtained from sulphide and sulphate. Sulphur occurs naturally in the environment and is the thirteenth most abundant element in the earth’s crust. It can be mined in its elemental form, although this method has declined over the last decade to less than 2% of world production. Today most elemental sulphur is obtained as a co-product recovered from oil and gas production in sweetening process.
The Greeks called it “theion”, the Romans “Sulfur” and the Anglo-Saxons “brimstone” – regardless of its name one thing is certain, Sulfur is vital for life.
Sulfur (also spelled Sulphur) is a chemical element with symbol S and atomic number 16.
We, as one of the main key players in supply and export of granular and lumps sulfur from Iran and Turkmenistan, are in position to supply and export sulfur for your esteemed company with competitive prices, flexible payment terms, and required packaging. Therefore, we invite all the traders, distributors and end-users to try us with our quality services and competitive prices.
Sulphur is the primary source in the production of sulphuric acid, the world’s most widely used chemical. Sulphuric acid is an essential intermediate in many processes in the chemical and manufacturing industries. Sulphuric acid also is used by the fertilizer industry to manufacture primarily phosphates, nitrogen, potassium, and sulphate fertilizers.
Sulfur reacts directly with methane to give carbon disulfide, used to manufacture cellophane and rayon. One of the direct uses of sulfur is in vulcanization of rubber, where polysulfide chains crosslink organic polymers. Large quantities of sulfites are used to bleach paper and to preserve dried fruit. Many surfactants and detergents (e.g. sodium lauryl sulfate) are sulfate derivatives. Calcium sulfate, gypsum, is mined on the scale of 100 million tonnes each year for use in Portland cement and fertilizers. Sulphur is increasingly used as a component of fertilizers. Sulfur can be used in agriculture and various industries such as plastics and many synthetic products, paper, paint, etc.
The increasing demand from the fertilizer manufacturing sector, coupled with increasing usage of sulfur for vulcanization of rubber are the main factors driving the growth of the global sulfur industry. Moreover, owing to the fall of crude prices, refineries across the globe are engaging in capacity expansion and other brownfield activities, leading to higher demand for sulfur. The demand for sulfur is also anticipated to be driven by the increasing usage of elemental sulfur as a major ingredient in the vulcanization process implemented to manufacture rubber. However, the enactment of stringent environmental regulations restricting the usage of sulfur and the high costs associated with its mining, are the key factors hindering the sulfur market growth.
The global market is segmented based on end-user applications, including agrochemicals, chemical processing, metal manufacturing, petroleum refining, and others. In 2016, the agrochemical industry occupied the largest share in terms of sulfur consumption. It is expected that the healthy growth of the phosphate fertilizer market will drive the global sulfur market over the forecast year.
Geographically, the market has been segmented into North America, South America, Europe, Asia-Pacific, and Middle East & Africa. Asia-Pacific represents the largest market in terms of consumption and production of sulfur. In the region, China is expected to be one of the largest producers of sulfur and sulfuric acid. Currently, the country imports more than a third of the global sulfur` trade. This group is able to provide sulfur with the highest quality in two kinds of granule and lump.
Sulphur Uses
– Sulfur is Used in Fireworks and Black Gunpowder
– as a fungicide , fumigant , and in the vulcanization of rubber
– in the manufacture of phosphate fertilizers
– in the treatment of certain akin diseases
– the most important compound is sulfuric acid
– Other important compounds include Sulfur dioxide , used as a bleaching agent , disinfectant and refrigerant
Sulfur Application
Agricultural fertilizer use: sulfur is an essential plant nutrient needed for the production of amino acids which in turn make up proteins.
Pure elemental sulfur is a vital secondary macronutrient that can be applied to deficient soils or in places where it is necessary to lower pH for acid loving plants. It is granulated for easy application through any type spreader.
Sulfur is essential in the structural and enzymatic components in plants. Sulphur is a key component of some essential amino acids and is needed for protein synthesis. Chlorophyll synthesis also requires S.
Sulfur (Sulphur) is not readily translocated within plants, so all plants need a continuous supply of Sulphur from emergence to crop maturity. Therefore, in S-deficient plants, older leaves may appear more healthy, while newer leaves and tissue may have stunted growth and a lighter green or even yellow appearance.
Most compound fertilizers containing Sulphur also contain nitrogen, highlighting the close link between these two elements. Sulphur is part of an enzyme needed for nitrogen uptake and lack of it can severely hamper nitrogen metabolism. Together with nitrogen, sulphur enables the formations of amino acids required for protein synthesis. It is found in fatty acids and vitamins and has an important impact on quality and taste or smell of crops.
Non-agricultural uses:
It is widely used in many consumer products and industrial applications. It’s commonly converted to sulfate prior to use in rubber, textile, detergent and paper.
Sulfur Packing
Our Sulfur Mineral 20-30%, is offered in three different shapes, Lumps, Grinded and powder. Sulphur packing will be in Bulk, 25 KG PP bags or Jumbo Bags.
Urea
Urea Description

Urea (carbamide), readily produced from ammonia and carbon dioxide, is a very important chemical in the agricultural and the polymer industries.
Urea, also known as carbamide, is an organic compound with chemical formula CO(NH2)2. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic. Dissolved in water, it is neither acidic nor alkaline. introduction and scientific invention of Urea is identified as a life changing event in the history of agriculture. Urea N46% is the world’s most common nitrogen fertilizer and has been used uniformly in all the agricultural lands of the world. Never before this, agriculture had seen such booming heights in production as well as prosperity. Urea is neutral in pH and can adapt to almost all kinds of soils. It is a waste product formed naturally by metabolizing protein in humans as well as other mammals, amphibians and some fish. Urea is widely used in the agricultural sector both as a fertilizer and animal feed additive.
The last decade has seen urea (carbamide) as a superb replacement to ammonium nitrate and has unleashed new unsurpassed production records. The white, crystalline solid comprises of a proportionate compound mixture of more than one nutrients and contains 46% nitrogen. Farmers have identified with this chemical fertilizer as a great ladder to huge success in agro revolution. The nutrient composition rendered by urea enhances the productivity of the soil and enriches its nutrient constituency. Providing each plant with relevant elements needed, urea sustains plant life.
More than 90% of world industrial production of urea is destined for use as a nitrogen-release fertilizer. Urea has the highest nitrogen content of all solid nitrogenous fertilizers in common use. Therefore, it has the lowest transportation costs per unit of nitrogen nutrient. Urea fertilizers rapidly transform to the ammonium form in soils.
Worldwide, urea N46% is one of the most widely used dry granular sources of nitrogen. It is preferred by the fertilizer manufacturing industry since it is relatively easy to manufacture. Urea also has a high nitrogen content (46%), in comparison to other popular nitrogen sources (i.e. ammonium nitrate). On a ton for ton basis, urea contains 35% more nitrogen than ammonium nitrate. This has implications on the storage and transport of nitrogen fertilizer products. Carbamide is considered a relatively stable product to store and transport, and it is for this reason that the transportation of Carbamide is considered very cost effective in comparison to its most common alternative, ammonium nitrate.
Advantages of Carbamide Fertilizer
– Superior Nitrogen content
– Low production cost, as source is natural
– Non-flammable and risk-free storage
– Wide application range, for all types of crops and soils
– Neutral pH and harmless to crops and soil
In 2022, the potential supply of urea fertilizers is expected to reach 197 million metric tons. Increasing crop prices lead to increased fertilizer demands and has been especially noted in recent years in South Asia. Favorable weather also increases demand for fertilizers in major agricultural regions.
Urea Uses
Urea is the world’s most commonly used nitrogen fertilizer and indeed more of it is manufactured by mass than any other organic chemical. Containing 46% N, it is the most concentrated nitrogen fertilizer, and is readily available as free-flowing prills (granules). It is the cheapest form of nitrogen fertilizer to transport and it is also the least likely to ‘cake’. It is therefore favoured in developing countries.
While over 90% of urea produced is used as a fertilizer, it has other uses, which include the manufacture of the melamine, used in melamine-methanal resins. Urea itself also forms important resins.
An increasingly important use of Carbamide is in reducing air pollution from diesel engines in cars, buses and lorries. Diesel engines run at high temperatures and nitrogen and oxygen, from the air, are able to react together under these conditions to produce high concentrations of nitric oxide. One way to remove this pollutant is to allow it to react with ammonia to form nitrogen.
However it is not possible to use ammonia directly as it is too volatile and is poisonous. Instead a solution of urea in water is injected into the hot gases emerging from the engine in the exhaust. It is thermally decomposed to ammonia and carbon dioxide. This is the reverse of the process used to make ammonia:
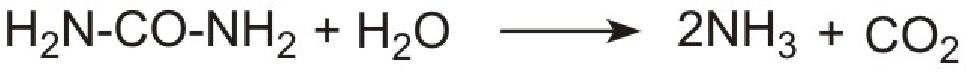
Unlike ammonia, urea is safe and easy to handle. The products, ammonia and carbon dioxide, together with the exhaust gases, are passed immediately over a catalyst in the exhaust system. Ammonia reduces the oxides of nitrogen (mainly nitric oxide), formed in the combustion processes, to nitrogen. The process is complex but the overall reaction can be represented thus:
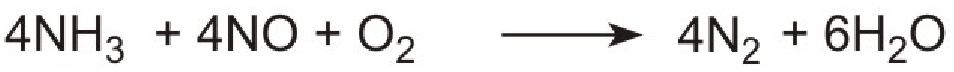
The system is known as Urea SCR (urea-based selective catalytic reduction) and can reduce pollution by nitrogen oxides to almost zero.
Annual Production of Carbamide
| World | 164 million tons |
| China | 62 million tons |
| India | 23 million tons |
| Middle East | 20 million tons |
| Rest of Asia | 18 million tons |
| FSU | 12 million tons |
| North America | 9.5 million tons |
| Europe | 9.5 million tons |
Urea Specifications
| Typical Chemical Analysis |
| Parameter Specification |
| Total Nitrogen Content, % 46.0 |
| Moisture content, wt. % 0.1 – 0.4 |
| Biuret, wt. % 0.85 – 1.5 |
| Conditioning agent, wt. % 0.30 – 0.60 |
| Typical Physical Properties |
| Parameter Specification |
| Bulk density, lb/ft3 48 – 52 |
| Angle of repose 28 – 32o |
| Size Guide Number (SGN) see note 1 240 – 320 |
| Uniformity Index (UI) see note 1 50 – 55 |
Packing of Carbamide N46
Packing in 50 kg polypropylene bags accepted up to 2000mt, for more quantities Bulk is available.
Linear Alkyl Benzene Sulphonic Acid
Sulfonic Acid (LABSA) Description

Sulfonic Acid (LABSA) is a batch of organic Sulfur compounds that are used as an anionic surfactant in most home detergents such as dishwashing detergents and washing powders. LABSA compound is used as a cleaning agent, foaming agent and sponge cleaner in more formulations. For the production of Sulfonic Acid, alkaline benzene linear sulfation is usually used. And its ingredients Linear Alkylbenzene, Oxygen, Sulfur and Citric Acid.
Linear Alkyl Benzene Sulphonic Acid is an anionic surfactant with molecules characterized by a hydrophobic and a hydrophilic group. They are non-volatile compounds produced by sulfonation. Linear alkylbenzene sulfonic acid are complex mixtures of homologues of different alkyl chain lengths (C10 to C13 or C14) and phenyl positional isomers of 2 to 5-phenyl in proportions dictated by the starting materials and reaction conditions, each containing an aromatic ring sulfonated at the para position and attached to a linear alkyl chain at any position with the exception of terminal one (1-phenyl).
The starting material LAB (linear alkyl benzene) is produced by the alkylation of benzene with n-paraffins
in the presence of hydrogen fluoride (HF) or aluminum chloride (AlCl3) as a catalyst, the latest is DETAL
process by UOP. LABSA is produced by the sulfonation of LAB with sulphuric acid in batch reactors.
Other sulfonation alternative reagents are oleum, diluted sulfur trioxide, chlorosulfonic acid and sulfamic
acid on falling film reactors.
Uses of Sulfonic Acid (LABSA)
High action of detergency, moistening, foaming, emulsion. Widely applied in a variety of detergents and emulsifiers, such as washing powder, daily-use chemical detergent, utensils detergents and textile industry of the cleaning agent, dye, electro plating industry, leather industry, degreasing agents and paper industry’s de-coloring agent. Household detergents including laundry powders, laundry liquids, dishwashing liquids and other household cleaners.
-It is used in anionic specialty formulations.
-In other industries such as textile industries, it is used as an mercerising or washing agent.
-It is used to increase the surface area of distempers.
-As main active matter in all forms of Detergents like Cake, Powder and Liquid formulations.
-As emulsifier and wetting agent in small quantity with other surfactants in Toilet soaps for foaming.
-In Pesticides to improve the quality of spray.
Sulfonic Acid (LABSA) Characteristics
- Chemical Formula : R-C6H4-SO3H
- CAS Number: 27176-87-0
- Active Matter (M=320) 96.0 % – 98.0%
- Specific Gravity (20°C) 1.050 – 1.060
- Free Oil < 2.5%
- Sulfuric Acid < 0.75%
- Preservation none
- Neutralization Index 13 (g NaOH 100/100 g)
- Color, APHA; ASTM D 1209 (Klett 5% AM) < 80
- Water Content 0.55%
LABSA Specifications
| Active ingredients | 97% (Min.) |
| Free Sulphuric Acid | 4-7% (Max) |
| Free Oil | 1% (Max) |
| Color Klett | 35 When packed (Max) |
| Density (gm/ml at 200c) | 1.07 |
| Appearance | Light Yellow-Brown Liquid |
| Viscosity | Low Viscosity |
| Mean Molecular Mass | Average 326 |
LABSA Packing

Sulfonic Acid or LABSA can be packed in 200 Kg PE Drums or 1 MT IBC Tanks.
80 Drums can be palletized and loaded in 1 full 20ft container.
20 IBC Tanks can be loaded in 1 full 20ft container.
White Spirit
Description of White Spirit

White spirit also known as: solvent naphtha, mineral spirit, varsol, mineral turpentine.
White spirit is a specialty refined product in the naphtha boiling range. It has a variety of uses including as an extraction solvent, a cleaner (paint thinner), or a degreaser. It is a common solvent in paints, lacquers, varnishes, aerosols, and asphalts.
The word “mineral” in “mineral spirits” or “mineral turpentine” is meant to distinguish it from distilled spirit (distilled directly from fermented grains and fruit) or from true turpentine (distilled tree resin).
Application of White Spirit
- Mineral spirits is a very powerful degreasing and cleansing agent for automobile and machinery part cleaning. The spirit eats away at oil and chemical grease and washes the part clean.
- Such spirits are even used to wash away dirt and grime from metallic objects and tools. Even dried paint, which accumulates on metal and thickens, can be dissolved and then washed off, on applying mineral spirits. This helps preserve the look and quality of the object, as opposed to scratching off the paint.
- They can be used as a solvent to erase mistakes made while painting.
- Wooden furniture which has been coated with a layer of lacquer, shellac or varnish, can be cleaned and polished with a small amount of mineral spirits. But first a small area of the furniture should be tested before complete use.
- It can be used to clean paint brushes, especially those used in heavy oils and paints.
- If sticky tape and price tags on glassware need to be removed, then dip a small ball of cotton in some mineral spirit and wipe off the sticker. Then rinse away the spirit.
- You can use mineral spirits on linoleum tiles to get rid of skid marks and scratches.
- Use mineral spirits to dissolve gum and other sticky resins, that are stuck to carpets or clothing.
- When combined with cutting oil, they are used as a lubricant for cutting threads.
Packing
White spirit packed in new or used 210 kg drums, iso tank, and IBC tank. Each 20-foot container takes 80 drums.
Specification
| CHARACTERISTICS | UNITS | RESULT | TEST METHOD |
| Density | Kg/m³ | 0.740 min | ASTM D1298 |
| I.B.P | °C | Min 60 | ASTM D86 |
| F.B.P | °C | Max 200 | ASTM D86 |
| Pour Point | °C | -30 | ASTM D93A |
| Color Say Bolt | — | 30 | ASTM D156 |
| Flash Point | °C | 42 min | ASTM D56 |
| Sulphur Total | ppm | 0.2 max | ASTM D4294 |
| Neutrality | — | Pass | ASTM BS.245 |
| Acidity of Residue | — | Pass | ASTM D1093 |
Caustic Soda
Caustic Soda Definition

Sodium hydroxide, also known as lye and Caustic Soda is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates NaOH·nH2O. The monohydrate NaOH·H2O crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available “sodium hydroxide” is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, it is frequently utilized alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.
NaOH or Sodium Hydroxide or Caustic Soda Or lye are thin white sheets that are moisture absorbent and soluble in water. It is derived from the evaporation of liquid caustic soda, which is itself produced through membrane technology.
Sodium hydroxide or Caustic Soda with NaOH chemical formula, a solid white solid with a melting temperature of 2°C and a density of 0.5 Perk profits can easily absorb moisture from the air and, therefore, have to be protected during transportation.
Chemical formula: NaOH
Analysis: 98±1 %
Scientific name: Sodium hydroxide
Industrial Name: caustic soda, lye
Caustic Soda Application
1. Used in the manufacture of pulp and paper, textiles, drinking water, soaps and detergents.
2. Used in as a drain cleaner.
3. Used in the paper industry.
4. Used in the manufacture of sodium salts and detergents.
5. Used for pH regulation.
6. Used in the Bayer process of Aluminum production.
7. Used in many scenarios where it is desirable to increase the alkalinity of a mixture, or to neutralize acids.
8. Used as an additive in drilling mud to increase alkalinity in bentonite mud systems.
9. Poor quality crude oil can be treated with sodium hydroxide to remove sulfurous impurities in a process known as caustic washing.
10. Widely used in pulping of wood for making paper or regenerated fibers.
11. Used to digest tissues, as in a process that was used with farm animals at one time.
12. Acts as an agent to make the solution alkaline, which aluminum can dissolve in.
13. Traditionally used in soap making.
14. Used as a catalyst for the transesterification of methanol and triglycerides.
15. washing or chemical peeling of fruits and vegetables, chocolate and cocoa processing, caramel coloring production, poultry scalding, soft drink processing, and thickening ice cream.
Characteristics
1. It is a white solid which has a melting point of 591K
2. It is a stable compound.
3. NaOH is bitter in taste and has a soapy feel to it.
4. It is highly soluble in water and moderately soluble in alcohol.
5. Sodium hydroxide is strongly alkaline in nature.
6. Boiling Point : 1388°C.
7. Melting Point : 318°C.
8. Easily soluble in cold water, hot water.
9. Density : 2.1 g/cm³ @ 25°C.
10. Vapor Pressure : 0 mmHg (approx)
Specification
| Product name | Caustic Soda Flakes 98% | |
| CAS | 1310-73-2 | |
| Standard | Result | |
| NaOH | 98% min | 98.15% |
| Na2CO3 | 0.90% max | 0.60% |
| NaCl | 0.15% max | 0.06% |
| Fe2O3 | 0.007% max | 0.006% |
Packing Of Caustic Soda
| Packing: | 25Kg Bags, 24MT Per 20′ FCL |
| Payment: | Negotiable |
| Minimum Order: | 1 x 20′ FCL |
Chemical Information
| Chemical Formula | NaOH |
| CAS number | 1310-73-2 |
| EC number | 215-185-5 |
| UN number | 1823 |
| Molar Mass | 40.0 g/mol |
| Color | White |
| Appearance | White Flakes |
| Melting point | 323 °C |
| Boiling point | 1390 °C |
| Solubility in Water | 111 g /100 ml at 20 °C |
| Synonyms: Sodium hydroxide, lye |
